-
-
- Seven-year-old mn fel DSH with chronic intermittent vomiting and diarrhea and weight loss. Cat has tenesmus. No abnormalities found on rectal palpation.Medial iliac lymph nodes-mildly reactive
- Fecal parasite testing is negative, no significant abnormalities notes on bloodwork.
- Abdominal ultrasound shows increased hepatic echogenicity and what appears to be reactive medial iliac lymph nodes. The gastrointestinal tract including the colon all appeared normal.
- US guided FNA of the liver was performed and results are pending.
- I recommended checking for anal gland mass (none found). Don’t see reactive MILN’s in cats very often. R/O infection, inflammation, neoplasia. Any other thoughts?
-
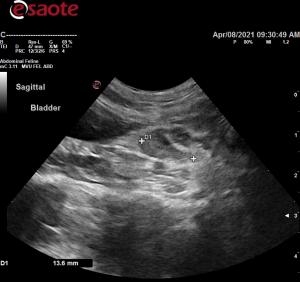

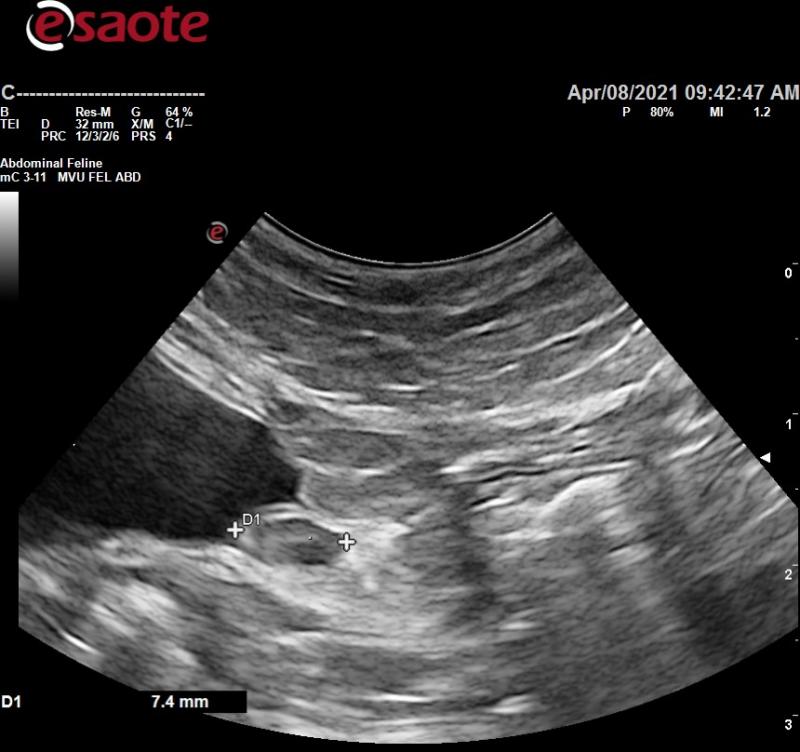
Comments
They look reactive to me wiht
They look reactive to me wiht pericapsular inflammation and not too hypoechoic. Infectious agents/lymphadenittis likely.
Good reference from ACVIM
Introduction
Abdominal lymphadenopathy, or masses that may be confused with enlarged lymph nodes, are common clinical findings in sick cats, either on physical examination or on ultrasonography. Many inflammatory, infectious, and neoplastic diseases can cause enlargement of regional lymph nodes in the abdomen, and assessment of lymph node size and cytological or histopathological characteristics may provide important information when more invasive diagnostic methods are not available. As with all sick cats, a meticulous history and physical examination are necessary to identify additional clinical abnormalities that may clarify the cause of the lymphadenopathy.
Causes of Lymphadenopathy
Lymph nodes that are palpable in normal cats and dogs include the mandibular, superficial cervical, axillary, superficial inguinal, and popliteal nodes. Lymphadenopathy is defined as enlargement of a solitary node, a regional group of nodes, or of all lymph nodes.1 Causes of lymph node enlargement in cats include reactive hyperplasia, resulting from proliferation of lymphocytes and plasma cells from antigenic stimulation; lymphadenitis, resulting from an influx of inflammatory cells due to local infection; and, neoplastic infiltration, either from primary lymphoid neoplasia or from infiltration from metastatic neoplastic disease.1
Differential Diagnosis of Abdominal Lymphadenopathy
Abdominal lymphadenopathy in cats may represent a response to a local disease process within the abdominal cavity, or may be a component of a systemic disease accompanied by generalized lymph node enlargement. Organomegaly, most often associated with small intestinal masses, but also due to splenomegaly or hepatomegaly, may accompany abdominal lymph node enlargement, complicating the accurate identification of lymphadenomegaly on palpation.
Reactive mesenteric lymph node hyperplasia is a common ultrasonographic abnormality, but less common physical finding, in cats with inflammatory bowel disease. The degree of thickening of the small intestine, and the degree of lymphadenomegaly, correlate well with the severity of the disease.2 Clinical signs include vomiting, diarrhea, weight loss, reduced appetite, and poor body condition and haircoat.
Intestinal tumors occur commonly in cats, accounting for 35% of all feline tumors.3 Approximately 74% of all feline intestinal tumors are the result of lymphoma, with intestinal adenocarcinomas being the cause of an additional 17% of intestinal neoplasia.3An abdominal mass is detected by palpation in 86% of cats with intestinal lymphoma, with lymphoblastic lymphoma being more likely to induce discrete masses. The small intestine is the most common site of lymphoma in cats, followed by the stomach, ileocecocolic junction, and lastly the colon.4 Diffuse intestinal thickening is more common in low-grade lymphocytic lymphoma. Concurrent mesenteric lymphadenopathy is noted ultrasonographically in 33-50% of cats with alimentary lymphoma, intestinal masses or thickening in about 40% of affected cats, and splenomegaly, hepatomegaly, or abdominal effusion in a smaller number of patients.4
Abdominal lymphadenopathy is a prominent feature of feline infectious peritonitis (FIP), especially the non-effusive form of the disease.5 In a series of 156 cats with FIP, 26 (16%) had a unusual manifestation of the disease, characterized by the presence of an isolated intramural intestinal mass, accompanied by local lymphadenomegaly.6 The nodular pyogranulomatous intestinal lesions were found most commonly in the colon (45%), ileocecocolic junction (31%), and small intestine (16%). Only 1 segment of intestine was affected in each cat. At surgery, many of the lesions were mistaken for primary intestinal neoplasia. Ultimately, all affected cats developed multisystemic signs of FIP, suggesting that the localized lesions were the result of temporary containment of infection by a partial cell-mediated immune response to the pathogenic coronavirus.6 In the author’s experience, this unusual manifestation of FIP is an important cause of palpable abdominal lymphadenomegaly in young cats with chronic disease and few other localizing signs.
Several other infectious diseases may be associated with abdominal lymphadenomegaly in cats, manifested either as regional lymphadenopathy or as part of a generalized lymph node enlargement.5 Histoplasma capsulatum infections in cats commonly cause peripheral and abdominal lymphadenopathy, and may be difficult to differentiate from those caused by non-effusive FIP or retrovirus infections in some patients. Clinical signs include weight loss, pyrexia, dyspnea, tachypnea, splenomegaly, hepatomegaly, anemia, and ocular, osseous, and cutaneous lesions. Acute infections, or recrudescences of latent infections, with Toxoplasma gondii may cause signs similar to those resulting from generalized histoplasmosis, and may include the development of focal intestinal masses and mesenteric lymphadenopathy. Infections with tuberculous mycobacteria (especially Mycobacterium bovis) in cats may cause primary intestinal disease with concurrent ileocecal and mesenteric lymphadenomegaly and splenic and hepatic involvement. Non-tuberculous mycobacterial (Mycobacterium avium complex) infections in cats may cause visceral disease, with thickened intestines, mesenteric lymphadenopathy, and hepatosplenomegaly as prominent signs. Methicillin-resistant staphylococcal infections occasionally may cause disseminated disease in cats, including abdominal lymphadenomegaly. The role of Ehrlichia species in causing overt disease in cats remains unclear; however, reported clinical signs are similar to those described earlier for histoplasmosis, with the exception of the pulmonary changes.5
Patient Evaluation
Signalment and History
Abdominal lymphadenomegaly in juvenile, or young adult, sick cats most likely is associated with an infectious disease; for example, FIP, histoplasmosis, or toxoplasmosis. Infiltrative intestinal diseases, whether inflammatory or neoplastic, are more likely to be the cause of visceral lymphadenopathy in mature or senior cats. Exceptions to these observations are common, however. Young pure-bred cats who were acquired from catteries within the past several months, and whose clinical signs include prominent abdominal lymph node enlargement, should be evaluated carefully for feline infectious peritonitis. The geographic location of where the cat has been living should be determined, because some diseases that cause abdominal lymphadenopathy (for example, infections caused by Histoplasma capsulatum and Francisella tularensis) are regional in their distribution.5
Physical Examination
A meticulous physical examination is an important part of the complete evaluation of the feline patient found to have abdominal lymphadenopathy on cursory examination or on ultrasonographic evaluation. Specific clinical signs associated with many diseases causing visceral lymphadenomegaly are described in the previous section. The presence of persistent fever suggests an infectious, inflammatory process, although this abnormality may be noted in neoplastic disease. A thorough ocular examination is an important and often overlooked component of the comprehensive evaluation of affected cats, because several infectious diseases causing lymphadenomegaly may induce inflammatory ocular changes that provide important diagnostic information. Detection of peripheral lymphadenopathy, in addition to visceral lymphadenomegaly, is suggestive of a disseminated infectious or neoplastic disease. Pain on palpation of enlarged abdominal lymph nodes is more likely to result from lymphadenitis than from reactive hyperplasia or neoplastic infiltration. Concurrent thickening of intestinal loops may be associated with inflammatory, infectious, or neoplastic infiltrative diseases.
Laboratory Evaluation
Comprehensive laboratory evaluation of cats with unexplained abdominal lymphadenopathy should include complete blood count, serum chemistries, urinalysis, tests for feline leukemia virus antigen and feline immunodeficiency virus antibody, and complete fecal parasitology. Additional serologic tests, based on patient signalment and clinical signs, include IgM and IgG titers for Toxoplasma gondii, baseline feline coronavirus antibody titer (to exclude infection), and urine antigen titers for Histoplasma capsulatum when no organisms have been identified. Additional polymerase chain reaction (PCR) testing on blood, lymph node aspirates, body cavity effusion, and feces may be indicated in coronavirus-seropositive cats in whom the suspicion of FIP is high. Serologic evaluation for Ehrlichia canis-like organisms may be performed when more common etiologic causes of systemic illness and visceral lymphadenomegaly have been excluded.
Serum cobalamin and serum folate levels, in combination with serum trypsin-like immunoreactivity levels, should be performed when concurrent weight loss and gastrointestinal signs (including thickened intestinal loops) are noted. In a recent study, 78% of cats with low-grade lymphocytic lymphoma were found to be hypocobalaminemic; mesenteric lymph node involvement was noted in 6 of 41 affected cats.7
Diagnostic Imaging
The ultrasonographic evaluation of canine and feline lymph nodes has been described recently.8 Ultrasound is considered more sensitive than survey radiography for detecting the presence of abdominal lymphadenomegaly; however, it is not sensitive for the identification of the etiologic cause of the lesions. Fine-needle aspirates or biopsies of lymph nodes are needed to clarify the ultrasonographic changes.9 Abdominal lymph nodes are classified as visceral or parietal, according to the anatomic area that they drain.8 Visceral lymph nodes include the jejunal nodes (also known as the cranial mesenteric nodes) and the right colic lymph nodes, both of which are often enlarged in inflammatory bowel disease or alimentary lymphoma. Ultrasonographic evidence of abdominal lymphadenopathy has been found in up to 50% of cats with alimentary lymphoma, with intestinal masses being identified sonographically in about 40% of affected patients.4
Ultrasonographic parameters that are useful to distinguish normal from abnormal lymph nodes include nodal size, shape, margin characteristics, echogenicity, echotexture, acoustic transmission, the presence of vascular flow and its distribution, and the measurement of vascular flow indices. Doppler evaluation of the abdominal lymph nodes of cats with alimentary lymphoma may show aberrant blood vessels entering the nodes in abnormal locations, in comparison to normal lymph nodes in which the hilus is the usual point of entry. On a practical basis, the parameters that provide the best predictability are the size and shape of the lymph node(s), the distribution of vessels within the node, and the pulsatility index that reflects vascular resistance within the node.8
Cytological Evaluation
The cytological evaluation of lymph node aspirates from cats and dogs has been described, including an algorithmic approach to the interpretation of cell types acquired during diagnostic testing.10 Small lymphocytes comprise 75-90% of the lymphocyte population obtained from normal lymph nodes, with medium-sized lymphocytes contributing 5-15%, and lymphoblasts 5%. Hyperplastic lymph nodes have the same respective lymphocyte counts as normal lymph nodes; however, nodal size is increased. Reactive lymphadenopathy is characterized by a small to moderate increase in the numbers of prolymphocytes, lymphoblasts, and plasma cells. Both of these cytological findings are considered to be the result of lymphoid proliferation in response to antigenic stimulation.10 Lymphadenitis is characterized by an increase in inflammatory cells, including neutrophils, macrophages, and eosinophils. The response is classified as primary if the lymph node is infected, and secondary if the node is not infected but is draining an infected lesion in its region.10 Lymphoblasts usually comprise at least 50% of the cell population in lymph nodes from patients with lymphoblastic lymphoma. Mesenteric lymph node aspirates from cats with low-grade lymphocytic lymphoma may yield a homogeneous population of small lymphocytes, in the absence of normal numbers of precursor cells. Biopsy and histopathological examination may be necessary to confirm the diagnosis of lymphoma. PCR testing to detect the presence of a clonal lymphocyte population also may be useful to confirm the cytological or histopathological findings.11
References
1. Schoeman JP. In Côté E (ed): Clinical Veterinary Advisor. Dog and Cat, Mosby, 2007, pp644.
2. Zoran DL. In Rand J (ed): Problem-Based Feline Medicine, 2006, Elsevier, pp734.
3. Selting KA. In Withrow SJ, Vail DM (eds): Withrow and MacEwen’s Small Animal Clinical Oncology, ed 4, 2007, Elsevier Saunders, pp491.
4. Vail DM. In Withrow SJ, Vail DM (eds): Withrow and MacEwen’s Small Animal Clinical Oncology, ed 4, 2007, Elsevier Saunders, pp733.
5. Green CE (ed): Infectious Diseases of the Dog and Cat, ed 3, 2006, Elsevier.
6. Harvey CJ, et al. J Am Vet Med Assoc 1996;209(6):1117.
7. Kiselow MA, et al. J Am Vet Med Assoc 2008;232(3):405.
8. Nyman HT, et al. Clin Tech Small Anim Pract 2007;22:128.
9. Forrest LJ. In Withrow SJ, Vail DM (eds): Withrow and MacEwen’s Small Animal Clinical Oncology, ed 4, 2007, Elsevier Saunders, pp 97.
10. Cowell RL, et al. Vet Clin Small Anim 2003;33:47.
11. Thrall MA. In Withrow SJ, Vail DM (eds): Withrow and MacEwen’s Small Animal Clinical Oncology, ed 4, 2007, Elsevier Saunders, pp112.
Thank you! Helps with
Thank you! Helps with differential diagnosis. Atypical case for me as just the medial iliac lymph nodes were affected but no anal or colic masses found. The liver aspirate came back as moderate to hepatic lipidosis. Odd because most recent chems had normal liver values.
… [Trackback]
[…] Informations on that Topic: members.sonopath.com/suspect-reactive-medilal-iliac-lymph-nodes-cat/ […]