– 8 year old F Lab Cross presented for lethargy and lactation; owner does not know if pet is spayed; rest of history unclear as many people involved in taking care of this pet
– rDVM took abdominal rads and was concerned about a possible pyloric mass
– u/s showed no evidence of a gastric mass; just full stomach and intestines with digesta (I wonder about polyphagia)
– 8 year old F Lab Cross presented for lethargy and lactation; owner does not know if pet is spayed; rest of history unclear as many people involved in taking care of this pet
– rDVM took abdominal rads and was concerned about a possible pyloric mass
– u/s showed no evidence of a gastric mass; just full stomach and intestines with digesta (I wonder about polyphagia)
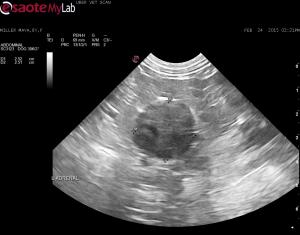
– a left adrenal mass (>2.5cm diameter) was detected that does not appear to be invading the renal blood vessels or the CVC (I followed the CVC from the right side in a cranial-caudal direction and flow was clean)
– right adrenal normal but may be a bit small for a patient this size (atrophy?)
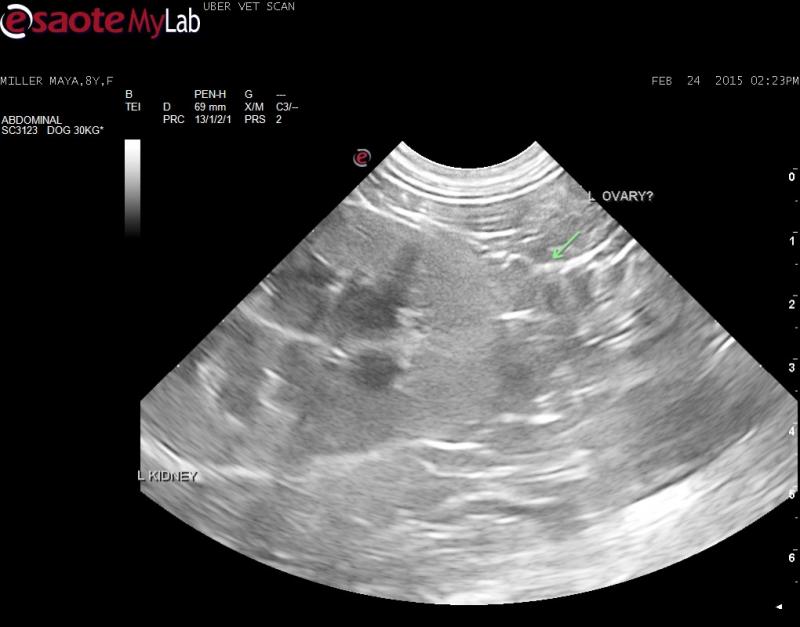
– I could not find a uterus or right ovary but found a structure caudal the left kidney that may be a left ovary?
Differentials: pseudopregnancy, retained ovarian remnant, hyperadrenocortism (secretory adrenal tumour), pheo, hypothyroidism (as another possible cause of lactation according to VIN)
What are your thoughts on the adrenal mass? Do you think the structure I am pointing to is a left ovary?
I have recommended: endocrine testing, BP measurement to start and to consider an expolatory to possibly remove a uterus if there or ovarian remnant and the adrenal mass as it appears to be resectable at this time
Comments
The adrenal mass is
The adrenal mass is resectable, may be progresterone secreting. You can run an adrenal profile at u of tennessee and see if progesterone is up but check bp and check for mets and cut.
Nice image set:
Here I gift you another sneak peak of curbside guide coming out shortly:
Adrenal Tumors
Description: An adrenal mass is suspected when the maximum width of the adrenal gland exceeds 1.5 cm, there is loss of normal architecture or shape, or the shape or size between the affected adrenal gland and the contralateral gland is asymmetrical. The latter comprise the initial criteria for diagnosis; however, a bulbous enlargement of the cranial or caudal pole of the adrenal gland is common in dogs with no adrenal pathology and can be misinterpreted as an adrenal mass. If the suspected mass is not precipitating obvious signs (i.e., aggressive behavior), then an abdominal ultrasound should be repeated to confirm that the mass is a consistent finding before pursuing further diagnostics or surgery. Large breeds (Poodles, German Shepherds, Retrievers, and Terriers) and females appear to be overrepresented in the clinical reviews of adrenal tumors. Adrenal tumors in cats are rare and insufficient information exists to characterize the disease.
Incidental adrenal lesions should be investigated clinically if discovered on ultrasound. Non-neoplastic adrenal lesions, such as cysts or granulomas, are very rare in dogs and cats, and the high incidence of metastatic lesions justifies a thorough hormonal screening as well as evaluation for non-adrenal neoplasms. Although incidental adrenal masses may appear to be nonfunctional at the time of diagnosis, it seems more likely that they are in fact subclinically functional. The diagnosis of functional adrenal tumors is discussed below; however, the identification of a nonfunctional, incidental adrenal mass creates a management dilemma.
Clinical Signs: Clinical signs attributable to adrenal tumors are dependent on hormone secretion type. Please see below.
Diagnostics: Cortical adrenal tumors, such as adenomas and adenocarcinomas, are responsible for 15-20% of hyperadrenocortical cases—what are commonly referred to as adrenal-dependent hyperadrenocortism (ADH)—in dogs. The remaining tumors are the result of pituitary-dependent secretions, which give rise to pituitary-dependent hyperadrenocorticism (PDH). PDH cases tend to demonstrate bilateral hypertrophy with excessive adrenal length and, probably more importantly, width. These enlarged adrenal glands do not invade surrounding vascular structures and are defined by overstimulation resulting from excessive ACTH secretion from the pituitary gland. Yet, ADH cases are usually unilateral (bilateral in 10-20% of cases), may invade the aorta on the left or the vena cava on the right, and metastasize to the liver and lungs most frequently. Practitioners must differentiate ADH masses from hyperplastic, non-functional, benign adrenal tumors, as well as pheochromocytomas. Thus, dynamic function tests (ex. LDDS, HDDS, ACTH stimulation, ACTH baseline, urine cortisol-creatinine ratio) are essential, as is conducting routine biochemistry (ALP is elevated in more than 90% of cases) and urinalysis (true polyuria/polydipsia [PU/PD] with USG < 1.020) to determine adequately the need for surgical intervention or aggressive medical therapy. It is important to assess the following: blood pressure for hypertension; oscillating hyper- and hypotensive episodes in cases of pheochromocytomas; urine protein-creatinine ratios; and serum antithrombin III to determine the risk for thromboembolism. Moreover, it is essential to evaluate the entire clinical picture and objective probabilities of possessing a true hyperadrenocorticism case. This further entails ruling out other sources of PU/PD, such as primary polydipsia, renal disease, electrolyte abnormalities, infections, and diabetes insipidus or mellitus.
Malignant or Benign, Functional or Non-Functional: How to Decide?
In some cases, it may be difficult to determine whether the mass is malignant or benign, functional or nonfunctional, prior to surgical removal and histopathological examination. A thorough review of the clinical signs, physical examination findings, routine blood work, urine tests, and appropriate hormonal tests should be conducted to determine the functional status of an incidental adrenal mass.
Malignancy is more often associated with larger masses. The larger the mass, the more likely metastasis has already occurred, in spite of a lack of detectable lesions on ultrasound and thoracic radiographs. Invasion of the mass into surrounding organs or blood vessels also supports malignancy, as does the detection of additional mass lesions with abdominal ultrasound and thoracic radiographs. Use of imaging modalities, such as CT and MRI, will likely provide additional data on the characteristics of specific adrenal lesions for use in diagnosis and treatment planning.
Ultrasonography is the primary instrument for assessing tumor size, aggressiveness, non-capsulated versus capsulated appearance, vascular invasion, and hepatic or other metastasis. Ideally, the patient will have fasted prior to the ultrasound; one may choose to administer an enema to enhance visibility around the ascending and descending colon. Ultrasound-guided biopsy or fine needle aspiration (FNA) may be possible on the larger masses, especially on the left side; however, adjacent vascular structures often prevent the feasibility of this procedure.
Diagnosis of the Functional Adrenal Mass:
A) Cortisol-Secreting: It is very rare that a patient with hyperadrenocorticism will have a repeatable urine specific gravity greater than 1.020, so it must be determined whether the patient is truly PU/PD. If yes, then dynamic function testing is appropriate. If the patient is not truly PU/PD, then a false positive result must be considered before treatment is initiated, as the resulting hypoadrenocorticism can be life threatening. Other causes of dysuria, such as occult urinary tract infection, must then be considered. The most common functional adrenal tumor identified in dogs and cats results in hyperadrenocorticism. Fifteen percent of ADH patients will be diagnosed with an adrenal mass, and 50% of these dogs will have malignant tumors.
B) Catecholamine-Producing: Pheochromocytoma is a tumor derived from the chromaffin cells of the adrenal medulla; it is relatively common in dogs, but quite rare in cats. These cases should be considered malignant until proven otherwise. Invasion/entrapment/compression of the caudal vena cava is common. Mural invasion or luminal narrowing of the aorta, renal vessels, adrenal vessels, and hepatic veins may also occur.
C) Aldosterone-Secreting (rare in dogs and cats):
D) Progesterone-Secreting: Although a functional tumor arising from the zona reticularis of the adrenal cortex could secrete excessive amounts of estrogen, progesterone, or testosterone, to date only progesterone-secreting adrenocortical tumors in cats have been documented.
E) Deoxycorticosterone-Secreting (rare):
F) 17-OH-progesterone-Secreting (rare):
Treatment: If hormonal tests for ADH and serum electrolytes are normal and clinical signs suggestive of pheochromocytoma are present, one can assume the adrenal mass is a pheochromocytoma and begin treatment with an alpha-adrenergic antagonist (ex. phenoxybenzamine at 0.25 mg/kg PO BID initially) for at least 2 weeks to prevent severe clinical manifestations of hypertension and promote a smooth anesthetic induction if adrenalectomy is planned. Adjustments to the dose are based on clinical response; an increase in the dose should be considered if clinical signs do not improve after 2 weeks of treatment. If hormonal tests for ADH and serum electrolyte concentrations are normal, clinical signs suggestive of pheochromocytoma are not present, but an adrenalectomy is nevertheless planned, one should still assume the adrenal mass is a pheochromocytoma and begin phenoxybenzamine treatment prior to adrenalectomy.
When a cortisol-producing adrenal tumor has been documented, medical therapy with trilostane (5-20mg/kg PO Q24hr) or mitotane (25-50 mg/kg PO Q24hr for 10 days, then every 4-7 days) should be considered.
The biggest dilemma is whether to perform an adrenalectomy if hormonal tests for hyperadrenocorticism and serum electrolyte concentrations are normal, and clinical signs and systemic hypertension suggestive of pheochromocytoma are not present.
An aggressive approach—adrenalectomy—is based on the assumption that the mass is malignant until proven otherwise and should be removed before metastasis has occurred. In theory, this approach would offer the best chance for long-term survival; however, the age of the patient, the size of the mass, the presence of concurrent diseases, the level of invasion into other organs, and the probability that metastases already exist should factor into the decision. Poor surgical candidates generally include: dogs compromised from the effects of hypercortisolis; older animals; animals with concurrent disease; those for whom invasion has been aggressive and surgical or post-surgical complications are likely; animals with very large masses that have likely already metastasized; and those with documented potential metastatic disease. In addition, adrenalectomy may not be indicated when the mass is small (< 3 cm diameter) and nonfunctional, and the patient is healthy. Reports suggest that there is an approximate 45% success rate of surgical resection of adrenal masses, with a positive prognosis inversely proportionate to tumor size.
In cases of concurrent hepatic nodular changes, liver biopsy samples can be obtained at surgery in cases of suspicious lesions visualized by ultrasound. Hyperadrenocorticism often causes benign nodular hyperplasia of the liver and should not be automatically interpreted as a sign of hepatic metastasis during ultrasonographic examination. Rather, suspect lesions should be confirmed and biopsied either at surgery or via ultrasound-guided FNA or core biopsy. Post-operative complications include delayed wound healing due to excessive corticoid circulation and wasting, hemorrhage, sepsis, and thromboembolism.
When surgery is a risk and a functional adrenal tumor has been documented, medical therapy, as outlined above, should be considered. Medical therapy will not impede metastatic events. An alternative approach in these cases is to determine the rate of growth of the mass by repeating abdominal ultrasounds initially at 2, 4, and 6 months. If the adrenal mass does not change in size, the time between ultrasound evaluations can be increased to every 4-6 months; however, if the adrenal mass is increasing in size, adrenalectomy should be considered.
Conclusion: Any incidentally discovered adrenal tumor warrants investigation into functionality and metastasis. The course of treatment for each case depends largely on which hormones are secreted by the adrenal tumor. Each case should be carefully evaluated on an individual basis before adrenalectomy is considered for aggressive tumors.
References:
Behrend EN, Kooistra HS, Nelson R, et al. Diagnosis of Spontaneous Canine Hyperadrenocorticism: 2012 ACVIM Consensus Statement (Small Animal). J Vet Intern Med 2013;27:1292–1304 .
Heorauf A, Reusch C. Ultrasonographic characteristics of both adrenal glands in 15 dogs with functional adrenocortical tumors. J Am Anim Hosp Assoc 1999;35(3):193-99.
Herrara MA, Mehl ML, Kass PH, et al. Predictive factors and the effect of phenoxybenzamine on outcome in dogs undergoing adrenalectomy for pheochromocytoma. J Vet Intern Med 2008;22(6):1333-39.
Nelson, RW. Diagnostic approach to the incidental adrenal mass. World Small Animal Veterinary Association World Congress, Granada, Spain, 3-5 October, 2002.
Syme HM, Scott-Moncrioeff JC, Treadwell NG, et al. Hyperadrenocorticism associated with excessive sex hormone production by an adrenocortical tumor in two dogs. J Am Vet Med Assoc 2001;219(12):1725-28.
Withrow, S. Management of endocrine neoplasia. World Small Animal Veterinary Association World Congress, Vancouver, BC, 8-11 August, 2001.
von Dehn BJ, Nelson RW, Feldman EC, Griffey SM. Pheochromocytoma and hyperadrenocorticism in dogs: six cases (1982-1992). J Am Vet Med Assoc 1995;207(3):322-24.
The adrenal mass is
The adrenal mass is resectable, may be progresterone secreting. You can run an adrenal profile at u of tennessee and see if progesterone is up but check bp and check for mets and cut.
Nice image set:
Here I gift you another sneak peak of curbside guide coming out shortly:
Adrenal Tumors
Description: An adrenal mass is suspected when the maximum width of the adrenal gland exceeds 1.5 cm, there is loss of normal architecture or shape, or the shape or size between the affected adrenal gland and the contralateral gland is asymmetrical. The latter comprise the initial criteria for diagnosis; however, a bulbous enlargement of the cranial or caudal pole of the adrenal gland is common in dogs with no adrenal pathology and can be misinterpreted as an adrenal mass. If the suspected mass is not precipitating obvious signs (i.e., aggressive behavior), then an abdominal ultrasound should be repeated to confirm that the mass is a consistent finding before pursuing further diagnostics or surgery. Large breeds (Poodles, German Shepherds, Retrievers, and Terriers) and females appear to be overrepresented in the clinical reviews of adrenal tumors. Adrenal tumors in cats are rare and insufficient information exists to characterize the disease.
Incidental adrenal lesions should be investigated clinically if discovered on ultrasound. Non-neoplastic adrenal lesions, such as cysts or granulomas, are very rare in dogs and cats, and the high incidence of metastatic lesions justifies a thorough hormonal screening as well as evaluation for non-adrenal neoplasms. Although incidental adrenal masses may appear to be nonfunctional at the time of diagnosis, it seems more likely that they are in fact subclinically functional. The diagnosis of functional adrenal tumors is discussed below; however, the identification of a nonfunctional, incidental adrenal mass creates a management dilemma.
Clinical Signs: Clinical signs attributable to adrenal tumors are dependent on hormone secretion type. Please see below.
Diagnostics: Cortical adrenal tumors, such as adenomas and adenocarcinomas, are responsible for 15-20% of hyperadrenocortical cases—what are commonly referred to as adrenal-dependent hyperadrenocortism (ADH)—in dogs. The remaining tumors are the result of pituitary-dependent secretions, which give rise to pituitary-dependent hyperadrenocorticism (PDH). PDH cases tend to demonstrate bilateral hypertrophy with excessive adrenal length and, probably more importantly, width. These enlarged adrenal glands do not invade surrounding vascular structures and are defined by overstimulation resulting from excessive ACTH secretion from the pituitary gland. Yet, ADH cases are usually unilateral (bilateral in 10-20% of cases), may invade the aorta on the left or the vena cava on the right, and metastasize to the liver and lungs most frequently. Practitioners must differentiate ADH masses from hyperplastic, non-functional, benign adrenal tumors, as well as pheochromocytomas. Thus, dynamic function tests (ex. LDDS, HDDS, ACTH stimulation, ACTH baseline, urine cortisol-creatinine ratio) are essential, as is conducting routine biochemistry (ALP is elevated in more than 90% of cases) and urinalysis (true polyuria/polydipsia [PU/PD] with USG < 1.020) to determine adequately the need for surgical intervention or aggressive medical therapy. It is important to assess the following: blood pressure for hypertension; oscillating hyper- and hypotensive episodes in cases of pheochromocytomas; urine protein-creatinine ratios; and serum antithrombin III to determine the risk for thromboembolism. Moreover, it is essential to evaluate the entire clinical picture and objective probabilities of possessing a true hyperadrenocorticism case. This further entails ruling out other sources of PU/PD, such as primary polydipsia, renal disease, electrolyte abnormalities, infections, and diabetes insipidus or mellitus.
Malignant or Benign, Functional or Non-Functional: How to Decide?
In some cases, it may be difficult to determine whether the mass is malignant or benign, functional or nonfunctional, prior to surgical removal and histopathological examination. A thorough review of the clinical signs, physical examination findings, routine blood work, urine tests, and appropriate hormonal tests should be conducted to determine the functional status of an incidental adrenal mass.
Malignancy is more often associated with larger masses. The larger the mass, the more likely metastasis has already occurred, in spite of a lack of detectable lesions on ultrasound and thoracic radiographs. Invasion of the mass into surrounding organs or blood vessels also supports malignancy, as does the detection of additional mass lesions with abdominal ultrasound and thoracic radiographs. Use of imaging modalities, such as CT and MRI, will likely provide additional data on the characteristics of specific adrenal lesions for use in diagnosis and treatment planning.
Ultrasonography is the primary instrument for assessing tumor size, aggressiveness, non-capsulated versus capsulated appearance, vascular invasion, and hepatic or other metastasis. Ideally, the patient will have fasted prior to the ultrasound; one may choose to administer an enema to enhance visibility around the ascending and descending colon. Ultrasound-guided biopsy or fine needle aspiration (FNA) may be possible on the larger masses, especially on the left side; however, adjacent vascular structures often prevent the feasibility of this procedure.
Diagnosis of the Functional Adrenal Mass:
A) Cortisol-Secreting: It is very rare that a patient with hyperadrenocorticism will have a repeatable urine specific gravity greater than 1.020, so it must be determined whether the patient is truly PU/PD. If yes, then dynamic function testing is appropriate. If the patient is not truly PU/PD, then a false positive result must be considered before treatment is initiated, as the resulting hypoadrenocorticism can be life threatening. Other causes of dysuria, such as occult urinary tract infection, must then be considered. The most common functional adrenal tumor identified in dogs and cats results in hyperadrenocorticism. Fifteen percent of ADH patients will be diagnosed with an adrenal mass, and 50% of these dogs will have malignant tumors.
B) Catecholamine-Producing: Pheochromocytoma is a tumor derived from the chromaffin cells of the adrenal medulla; it is relatively common in dogs, but quite rare in cats. These cases should be considered malignant until proven otherwise. Invasion/entrapment/compression of the caudal vena cava is common. Mural invasion or luminal narrowing of the aorta, renal vessels, adrenal vessels, and hepatic veins may also occur.
C) Aldosterone-Secreting (rare in dogs and cats):
D) Progesterone-Secreting: Although a functional tumor arising from the zona reticularis of the adrenal cortex could secrete excessive amounts of estrogen, progesterone, or testosterone, to date only progesterone-secreting adrenocortical tumors in cats have been documented.
E) Deoxycorticosterone-Secreting (rare):
F) 17-OH-progesterone-Secreting (rare):
Treatment: If hormonal tests for ADH and serum electrolytes are normal and clinical signs suggestive of pheochromocytoma are present, one can assume the adrenal mass is a pheochromocytoma and begin treatment with an alpha-adrenergic antagonist (ex. phenoxybenzamine at 0.25 mg/kg PO BID initially) for at least 2 weeks to prevent severe clinical manifestations of hypertension and promote a smooth anesthetic induction if adrenalectomy is planned. Adjustments to the dose are based on clinical response; an increase in the dose should be considered if clinical signs do not improve after 2 weeks of treatment. If hormonal tests for ADH and serum electrolyte concentrations are normal, clinical signs suggestive of pheochromocytoma are not present, but an adrenalectomy is nevertheless planned, one should still assume the adrenal mass is a pheochromocytoma and begin phenoxybenzamine treatment prior to adrenalectomy.
When a cortisol-producing adrenal tumor has been documented, medical therapy with trilostane (5-20mg/kg PO Q24hr) or mitotane (25-50 mg/kg PO Q24hr for 10 days, then every 4-7 days) should be considered.
The biggest dilemma is whether to perform an adrenalectomy if hormonal tests for hyperadrenocorticism and serum electrolyte concentrations are normal, and clinical signs and systemic hypertension suggestive of pheochromocytoma are not present.
An aggressive approach—adrenalectomy—is based on the assumption that the mass is malignant until proven otherwise and should be removed before metastasis has occurred. In theory, this approach would offer the best chance for long-term survival; however, the age of the patient, the size of the mass, the presence of concurrent diseases, the level of invasion into other organs, and the probability that metastases already exist should factor into the decision. Poor surgical candidates generally include: dogs compromised from the effects of hypercortisolis; older animals; animals with concurrent disease; those for whom invasion has been aggressive and surgical or post-surgical complications are likely; animals with very large masses that have likely already metastasized; and those with documented potential metastatic disease. In addition, adrenalectomy may not be indicated when the mass is small (< 3 cm diameter) and nonfunctional, and the patient is healthy. Reports suggest that there is an approximate 45% success rate of surgical resection of adrenal masses, with a positive prognosis inversely proportionate to tumor size.
In cases of concurrent hepatic nodular changes, liver biopsy samples can be obtained at surgery in cases of suspicious lesions visualized by ultrasound. Hyperadrenocorticism often causes benign nodular hyperplasia of the liver and should not be automatically interpreted as a sign of hepatic metastasis during ultrasonographic examination. Rather, suspect lesions should be confirmed and biopsied either at surgery or via ultrasound-guided FNA or core biopsy. Post-operative complications include delayed wound healing due to excessive corticoid circulation and wasting, hemorrhage, sepsis, and thromboembolism.
When surgery is a risk and a functional adrenal tumor has been documented, medical therapy, as outlined above, should be considered. Medical therapy will not impede metastatic events. An alternative approach in these cases is to determine the rate of growth of the mass by repeating abdominal ultrasounds initially at 2, 4, and 6 months. If the adrenal mass does not change in size, the time between ultrasound evaluations can be increased to every 4-6 months; however, if the adrenal mass is increasing in size, adrenalectomy should be considered.
Conclusion: Any incidentally discovered adrenal tumor warrants investigation into functionality and metastasis. The course of treatment for each case depends largely on which hormones are secreted by the adrenal tumor. Each case should be carefully evaluated on an individual basis before adrenalectomy is considered for aggressive tumors.
References:
Behrend EN, Kooistra HS, Nelson R, et al. Diagnosis of Spontaneous Canine Hyperadrenocorticism: 2012 ACVIM Consensus Statement (Small Animal). J Vet Intern Med 2013;27:1292–1304 .
Heorauf A, Reusch C. Ultrasonographic characteristics of both adrenal glands in 15 dogs with functional adrenocortical tumors. J Am Anim Hosp Assoc 1999;35(3):193-99.
Herrara MA, Mehl ML, Kass PH, et al. Predictive factors and the effect of phenoxybenzamine on outcome in dogs undergoing adrenalectomy for pheochromocytoma. J Vet Intern Med 2008;22(6):1333-39.
Nelson, RW. Diagnostic approach to the incidental adrenal mass. World Small Animal Veterinary Association World Congress, Granada, Spain, 3-5 October, 2002.
Syme HM, Scott-Moncrioeff JC, Treadwell NG, et al. Hyperadrenocorticism associated with excessive sex hormone production by an adrenocortical tumor in two dogs. J Am Vet Med Assoc 2001;219(12):1725-28.
Withrow, S. Management of endocrine neoplasia. World Small Animal Veterinary Association World Congress, Vancouver, BC, 8-11 August, 2001.
von Dehn BJ, Nelson RW, Feldman EC, Griffey SM. Pheochromocytoma and hyperadrenocorticism in dogs: six cases (1982-1992). J Am Vet Med Assoc 1995;207(3):322-24.
Thanks for the info and
Thanks for the info and speedy response!
Any thoughts on the ovary suspect?
Thanks for the info and
Thanks for the info and speedy response!
Any thoughts on the ovary suspect?
On the still I can’t really
On the still I can’t really comment one way or the other. Do you have a video of that region?
On the still I can’t really
On the still I can’t really comment one way or the other. Do you have a video of that region?
Sorry – no clip. I guess the
Sorry – no clip. I guess the dog needs to be explored anyway so that region can be looked at then. Thanks again.
Sorry – no clip. I guess the
Sorry – no clip. I guess the dog needs to be explored anyway so that region can be looked at then. Thanks again.
You could consider performing
You could consider performing both an anti-mullerian test and measuring a progesterone level. Dogs with retained ovaries in diestrus will give a false negative on the anti-mullerian test. However, if they are in diestrus, you should get a progesterone level > 0.2 ng/ml. Performing these tests prior to exploratory surgery will let the surgeon know if they need to look for and remove an ovary/ovarian remnant(s).
You could consider performing
You could consider performing both an anti-mullerian test and measuring a progesterone level. Dogs with retained ovaries in diestrus will give a false negative on the anti-mullerian test. However, if they are in diestrus, you should get a progesterone level > 0.2 ng/ml. Performing these tests prior to exploratory surgery will let the surgeon know if they need to look for and remove an ovary/ovarian remnant(s).